Abstract
Acute myeloid leukemia (AML) cells are highly dependent on oxidative phosphorylation (OxPhos) for survival and continually adapt to fluctuations in nutrient and oxygen availability in the bone marrow (BM) microenvironment. The first-in-class OxPhos inhibitor IACS-010759 (Molina, Nat. Medicine 2018) is currently in Phase 1 clinical trial in AML. To identify biomarkers of sensitivity or resistance to OxPhos inhibition, we performed Cap Analysis of Gene Expression analysis (CAGE) in primary AML samples (11 sensitive and 3 resistant to IACS-010759) and 6 AML cell lines (OCI-AML3, MOLM13, THP-1, U937, MV4;11, HL60). CAGE identified 41 gene transcripts that were significantly higher in IACS-010759 resistant primary AML samples than in sensitive samples (fold change >3.0, FDR <0.05, EdgeR). Among these, mitochondria metabolism related genes ATP1B2, GCLC, SLC25A39 and AKR1A1 showed higher baseline expression in IACS-resistant MOLM13 cells compared to sensitive OCI-AML3 cells, suggesting that the basal metabolic capacity affects sensitivity to OxPhos inhibition. Indeed, MOLM-13 showed markedly higher baseline oxygen consumption rates (OCR) and more prominent elevation of compensatory glycolytic proton efflux rate (glycoPER) after IACS-010759 treatment than OCI-AML3 cells (Seahorse XF Analyzer). The simultaneous inhibition of glycolysis by 2-deoxy-D-glucose (2-dG) and OxPhos induced synergistic cytotoxicity and proliferation inhibition (combination index, CI: 0.04, 0.81 OCI-AML3, < 0.01, 0.29 MOLM13).
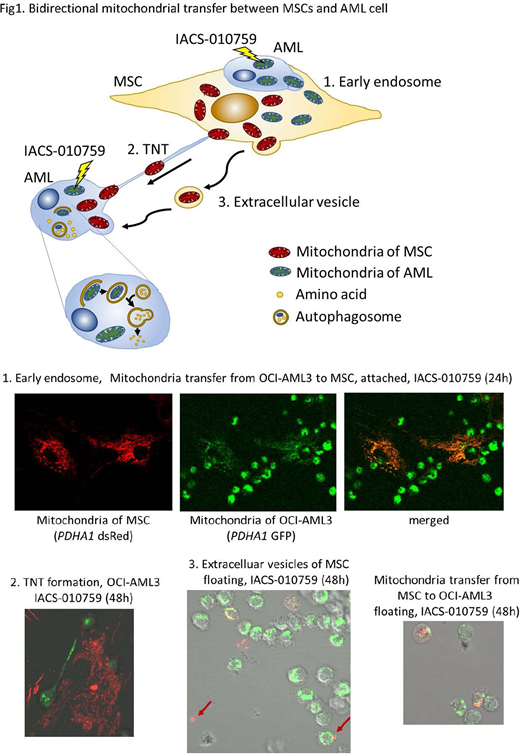
We next studied OxPhos inhibition under conditions mimicking BM microenvironment, by co-culturing AML cells with healthy BM-derived mesenchymal stem cells (MSC). Anti-proliferative effects of IACS-010759 were reduced by co-culture with MSC (IC50 0.01nM vs 2.1nM OCI-AML3, 65.4nM vs >10µM MOLM13). MSC co-culture increased OCR and glycoPER, and IACS-010759 decreased OCR / increased glycoPER in both cell lines. To determine the mechanism of resistance to IACS-010759 under MSC co-culture conditions, we studied the mitochondrial exchange between AML cells and MSC. We observed the bidirectional mitochondrial transfer between MSCs and AML cells. Using AML and MSC cells stably infected with mitochondria-targeted PDHA1 (GFP in AML, dsRed in MSC) to visualize the mitochondria, we found that IACS-010759 treatment facilitated transfer of AML-derived mitochondria into the adhered MSCs (24h), and MSC-derived mitochondria transferred to floating AML cells (48-72h) along with formation of tunneling nanotubes (TNTs) of AML cells, in sensitive OCI-AML3 cells (PDHA1-GFP/DsRed double positive %, control vs IACS; 9.0% vs 23.3 %). Mitochondria transfer from MSCs to AML cells was minimally observed in MOLM13 cells with elevated basal OxPhos (4.0% vs 5.0%).
We next investigated IACS-010759-induced metabolic changes in AML cells interacting with bone marrow (BM) stromal cells. Capillary electrophoresis-time-of-flight mass spectrometry (CE-TOFMS) metabolite analysis further detected that IACS-010759 suppressed multiple anaplerotic amino acids in OCI-AML3 cells including glutamine and glutamic acid, which was reversed by MSC co-culture along with increase in intra-autophagosomal LC3-II in OCI-AML3 cells. These findings were not observed in MOLM13. Microenvironment-induced activation of glutamine metabolism and autophagy-associated amino acid metabolism could be additional compensatory mechanisms in response to OxPhos inhibition by IACS-010759 in BM microenvironment.
Taken together, basal metabolic energetic capacity including elevated OxPhos and high compensatory glycolysis confer the AML-intrinsic resistance to IACS-010759, which can be reversed by simultaneous glycolysis inhibition. BM microenvironment facilitates secondary resistance to OxPhos inhibition through modulation of amino acid metabolism and direct mitochondrial transfer. Inhibition of these processes could enhance the anti-AML efficacy of OxPhos inhibition.
Andreeff:AstraZeneca: Research Funding. Konopleva:Stemline Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal